Sunday, July 14, 2013
Sunday, June 9, 2013
Avian influenza A H7N9
Being a daddy and homeowner (maintenance, repairs) have taken priority over writing entries for this blog, but I'm not complaining!
I've been interested in the H7N9 epidemic in China since is began two months ago. I had considered writing a post on H7N9 for several weeks. I've been asked to write an article for a health department newsletter, which will cover the basics of what clinicians should know about H7N9, but I'd like to go into more detail here.
Influenza A H7N9 was first detected on March 30th
in specimens from three people in China who died from severe respiratory
disease. Within a month there were 128
cases and 24 deaths from H7N9 in China. As of May 31st, there
have been 132
cases and 37 deaths. Most of cases and deaths were older adults and most
had recently had contact with live chickens or had been in live bird markets. One
cases of H7N9 influenza was detected outside of China, but he had recently
traveled to China.
H7N9 is the first H7 influenza detected in human in Asia and
the first N9 subtype virus ever detected in humans.
It occurred to me that I haven't written a post on avian
influenza. In my previous post, what
are H and N? I briefly discussed the diversity of hemagglutinin and
neuraminidase, the glycoproteins on the surface of influenza viruses. I also
mentioned that the ability of influenza viruses to infect humans depends upon
how well the hemagglutinin on the virus surface is adapted to receptors in our respiratory
tract. There are a couple of things I didn't discuss in that post that I
think are important to understand about influenza A viruses.
As I mentioned in my previous post, there are 16
hemagglutinins and 9 neuraminidases. Hemagglutinin is the molecule on the surface
of influenza viruses that attaches to the cell membrane and allows the virus to
enter the cell. The virus then uses the cell's protein synthesis mechanisms to
create new copies of itself. Neuraminidase is the molecule on the surface of
the virus that allows the newly created viruses to escape the infected cell.
 |
CDC, 2012
|
 |
Influenza A viruses have a segmented genome. The gene segments can reassort in a cell infected with two viruses. This can result in the creation of new (novel) influenza viruses. The danger of reassortment is that a pandemic virus can be created that is easily spread from person-to-person and to which most people have little or no immunity.

Like 2009 H1N1, H7N9 is a triple reassortant; that is, it has genes from three different influenza viruses. Unlike 2009 H1N1, there is no conclusive evidence that H7N9 is transmitted from person-to-person.
For sustained human-to-human transmission to occur, an
influenza virus must be adapted to α2-6 linked sialic acid receptors on cells
in the upper part of the human respiratory tract. Avian influenza viruses are
adapted to α2-3 linked sialic acid receptors found in birds. Humans have α2-3
linked sialic acid receptors in the lower part of our airway, so humans can be
infected with avian influenza viruses, but they are not easily transmitted from
one person to another. Pigs have both α2-6 linked and α2-3 linked sialic acid
receptors, so pigs are often the intermediate host for avian influenza viruses
that become human influenza viruses, but H7N9 has not been found in pigs.
Genetic analysis of the hemagglutinin gene in H7N9 suggests that it may be
better adapted to human sialic acid receptors.
Avian influenza viruses circulate widely in poultry and are
frequently responsible for economic losses when infected birds either die or
are culled. H7 avian influenza viruses have sporadically infected poultry
workers or people who are involved with culling infected flocks in North
America and Europe. The most frequent clinical manifestation of human H7
influenza virus infection is conjunctivitis
(pinkeye). Mild respiratory illness is also common. Although a few mild H7N9
infections have been identified, the most cases have been associated with
severe respiratory disease requiring hospitalization and admission to an intensive care unit
(ICU).
Before the H7N9 epidemic in China, the largest outbreak of
an H7 virus was in The Netherlands in 2003. Eighty three people were found to
be infected with H7N7. Seventy eight of them had conjunctivitis, seven had mild
respiratory disease, and one 57-year-old veterinarian died from acute
respiratory distress syndrome (ARDS).
In contrast to H5N1, which is a highly pathogenic avian
influenza virus (HPAI), H7N9 is a low pathogenic avian influenza virus (LPAI).
In this case, pathogenicity refers to the viruses' ability to cause disease in
bird, not humans. It's easy to tell when a flock is infected with an HPAI like
H5N1 because there are a lot of sick and dead birds. H7N9 appears to cause very
mild or no illness in birds. This makes H7N9 more dangerous because humans can
be exposed to infected birds without any easily recognizable indication that
the birds are infected.
In response to the H7N9 epidemic, local and national
authorities in China closed live bird markets in the affected provinces. The
number of cases has decreased since then and emergency
responses ended May 28th. The reservoir for the virus has not been
identified, but the virus has not been detected in poultry farms.
Whatever happened to H5N1 that we heard so much about ten
years ago? It's still out there and still killing people. So far this year
there have been 20
cases and 15 deaths caused by avian influenza A H5N1.
Another one to watch: H9 avian influenzas.
More information:
Centers for Disease Control and Prevention
Center for Infectious Disease Research & Policy
World Health Organization
References:
Belser, J. A., Bridges, C. B., Katz, J. M., & Tumpey, T.
M. (2009). Past, present, and possible future human infection with influenza
virus A subtype H7. Emerging Infectious
Diseases, 15(6), 859-865. http://wwwnc.cdc.gov/eid/article/15/6/09-0072_article.htm.
Fouchier, R. A. M., Schneeberger, P. M., Rozendaal, F. W.,
Broekman, J. M., Kemink, S. A. G., Munster, V. et al. (2004). Avian influenza A
virus (H7N7) associated with human conjunctivitis and a fatal case of acute
respiratory distress syndrome. PNAS,
101(5), 1356-1361. http://www.pnas.org/content/101/5/1356.full.
Gao, H-V., Lu, H-Z., Cao, B., Du, B., Shang, H., Gan, J-H.
et al. (2013). Clinical findings in 111 cases of influenza A (H7N9) virus
infection. New England Journal of Medicine, DOI: 10.1056/NEJMoa1305584. http://www.nejm.org/doi/full/10.1056/NEJMoa1305584.
Gao, R., Cao, B., Hu, Y., Feng, Z., Wang, D. et al. (2013).
Human infection with a novel avian-origin influenza A (H7N9) virus. New England Journal of Medicine, 368(20), 1888-1897.
http://www.nejm.org/doi/full/10.1056/NEJMoa1304459.
Hu, Y., Lu, S., Song, Z., Wang, W., Hao, P., Li, J. et al.
(2013). Association between adverse clinical outcome in human disease caused by
novel influenza, A H7N9 virus and sustained viral shedding and emergence of
antiviral resistance. Lancet, DOI:
10.1016/S0140-6736(13)61125-3. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61125-3/abstract.
Li, Q., Zhou, L., Zhou, M., Chen, Z., Li, F., Wu, H. et al.
(2013). Preliminary report: epidemiology of the avian influenza A (H7N9)
outbreak in China. New England Journal of
Medicine, DOI: 10.1056/NEJMoa1304617. http://www.nejm.org/doi/full/10.1056/NEJMoa1304617.
Murhekar, M., Arima, Y., Horby, P., Vandemaele, K. A. H.,
Vong, S., Zijian, F. et al. (2013). Avian influenza A (H7N9) and the closure of
live bird markets. Western Pacific
Surveillance and Response Journal, 4(2), doi: 10.5365/wpsar.2013.4.2.008. http://www.wpro.who.int/wpsar/volumes/04/2/2013_PE_EMT_Murhekar/en/index.html.
Osterholm, M. T., Ballering, K. S., & Kelley, N. S.
(2013) Major challenges in providing an effective and timely pandemic vaccine
for influenza A (H7N9). JAMA, doi:10.1001/jama.2013.6589.
http://jama.jamanetwork.com/article.aspx?articleid=1686871.
Treanor, J. J. (2009). Influenza viruses, including avian
influenza and swine flu. In G. L. Mandell, J. E. Bennett, & R. Dolin
(Eds.). Mandell, Douglas, and Bennett’s
principles and practice of infectious diseases. (7th Ed.) [Electronic
version].
Uyeki, T. M. & Cox, N. J. (2013). Global concerns
regarding novel influenza A (H7N9) virus infections. New England Journal of Medicine, 368(20), 1862-1864. http://www.nejm.org/doi/full/10.1056/NEJMp1304661.
Xu, C., Havers, F., Wang, L., Chen, T., Shi, J, Wang, J. et
al. (2013). Monitoring avian influenza A (H7N9) virus through national
influenza-like illness surveillance, China. Emerging
Infectious Diseases, 19(8), DOI: 10.3201/eid1908.130662. http://wwwnc.cdc.gov/eid/article/19/8/13-0662_article.htm.
Sunday, March 10, 2013
Travel vaccines
 |
| Future world traveler |
Traveler's health includes
much more than infectious disease. More travelers die from cardiovascular
disease, motor vehicle accidents, and drowning than die from infectious
diseases. The most common infectious disease of travelers is traveler's diarrhea, which is usually self-limiting, but can be serious and can put a
vacationer out of commission for a substantial portion of a long-awaited trip.
Last month Mogens Jensenius and colleagues published a study of potentially life-threatening tropical diseases in travelers. Between June 1996 and August 2011 82,825
illnesses in travelers were reported to GeoSentinel. Of those,
3,666 (4.4%) had potentially life-threatening diseases. Falciparum malaria was by far the most frequently
reported potentially life-threatening disease.
I can't adequately address all of the aspects of traveler's
health here. I've included some resources below. Please note that immunization
recommendations and requirements for travel change, so I strongly recommend
consulting a travel medicine specialist at
least one month before your planned departure date.
There are three categories of travel immunizations: routine,
recommended, and required.
Routine immunizations
Outbreaks of diseases that have been eliminated from the U.S. continue to occur throughout the world, including outbreaks
of vaccine-preventable disease in wealthy countries:
All travelers should be up to date on all routine immunizations. Depending on age and destination, some travelers may require
booster doses.
Influenza
Unlike temperate
climates, there is no "flu season" in the tropics. Influenza is
transmitted year round, so travelers to tropical climates should receive an annual flu vaccine
at least two weeks prior to travel.
Measles, mumps, and
rubella
In 1989 the CDC's Advisory Committee on Immunization
Practices changed its recommendations from a single dose of measles, mumps, and rubella vaccine (MMR) to two doses. Adults who have received one dose
of MMR should receive a second dose. This recommendation includes
travelers to Europe. Nearly half of the measles cases imported into the U.S. in
2011 came from Europe.
Inactivated poliovirus
vaccine
Although wild poliovirus transmission has been interrupted
in all but three countries (Afghanistan, Nigeria, and Pakistan), a risk of
poliovirus infection to travelers exists in countries in which
- Oral poliovirus vaccine (OPV) is used
- Vaccine-associated paralytic polio (VAPP)
- Vaccine-derived polioviruses are circulating (cVDPV)
- Areas with inadequate immunization coverage
Recommended immunizations
Vaccines recommendations depend on the epidemiology of the
disease in the destination country and the age and health status of the
traveler. Recommended vaccines include hepatitis A, hepatitis B,
Japanese encephalitis, meningococcal,
pneumococcal,
typhoid,
varicella (chickenpox), and yellow fever
vaccines.
Notice that several of these vaccines are included
in the current ACIP childhood immunization schedules and may be
required for school admission. I'm only going to discuss the more "exotic"
diseases here.
Meningococcal vaccine
Meningococcus (Neisseria
meningitidis) is transmitted by respiratory droplets. As the name suggests,
meningococcus can cause bacterial meningitis (headache, photophobia,
stiff neck, confusion, and permanent neurological damage). Meningococcemia
(meningococcus in the blood) causes petechial rash, shock,
loss of fingers and limbs, and can be rapidly fatal.
Risk factors
for transmission include crowding, poverty, and smoking. Some people can carry
meningococcus without developing meningococcal disease (asymptomatic nasopharyngeal
carriage). Outbreaks usually occur in settings in which people live in close
contact with each other. In this country, those settings include college dormitories and military barracks.
Major meningococcal epidemics occur every 5 to 10 years in
the African Meningitis Belt. Epidemics occur during hot, dry, dusty seasons and
end at the beginning of the rainy season. I worked in Ethiopia during a
meningococcal epidemic. During the first three months of the epidemic we had no
vaccine and could only educate people about the symptoms of meningitis and
treat those who came to the health center.
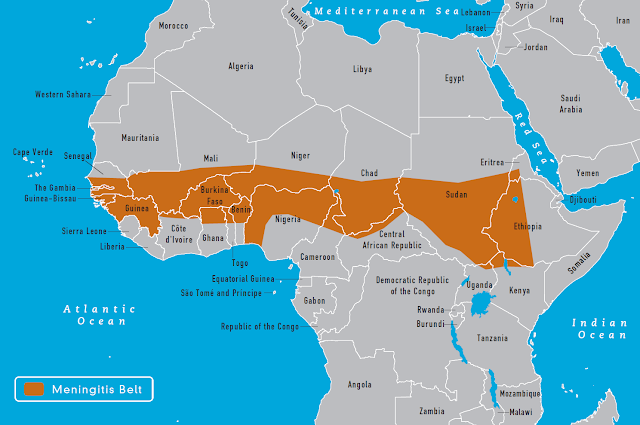 |
| CDC, 2012 |
Typhoid fever is caused by the bacteria, Salmonella enterica, subspecies Typhi.
Typhoid is transmitted by the fecal-oral route in areas with substandard
sanitation. There are several Salmonella
species that cause symptoms that are clinically indistinguishable from typhoid.
Symptoms include fever, relative bradycardia, abdominal pain, constipation
and/or diarrhea, headache, and mental status changes. Typhoid can be fatal if
not treated and resistance to antibiotics is becoming more common.
There are two typhoid vaccines licensed in the U.S.: Vivotif®,
a live attenuated oral typhoid vaccine and Typhim Vi®, an injectable
polysaccharide vaccine. Neither of these vaccines prevent non-typhoidal
Salmonella infections.
Japanese encephalitis
Like dengue, West Nile virus, and yellow fever, Japanese
encephalitis (JE) is caused by a flavivirus (flavi- "yellow") and is transmitted by mosquitoes. Most
infections are asymptomatic. About 1% of people infected with the Japanese
encephalitis virus will develop encephalitis. Symptoms include fever, headache,
lethargy, movement disorders, mental status changes, seizures, and focal
neurological deficits. Case fatality is around 20 to 30% and 30 to 50% of
survivors will be left with residual neurological deficits.
Most travelers to endemic countries are not at risk. The
risk is low in urban areas. Travelers who have long stays in or frequent
visits to rural/farming areas may be at risk. The JE vaccine available in the
U.S. is licensed for people 17 years of age and older. The vaccine is given in
two doses 28 days apart; the second dose must be given at least 1 week before
arriving in an endemic area. There is no JE vaccine available in the U.S. for
children 16 years of age and younger.
 |
| CDC, 2012 |
Yellow fever is endemic in parts of Africa and South
America. It was introduced to the United States via the slave trade and caused
major epidemics on the Eastern Seaboard and Mississippi Valley. Mosquito
control eliminated the disease from the U.S. The last major epidemic was in New
Orleans in 1905. Yellow fever is maintained in the wild in monkeys.
Most yellow fever virus infections are self-limiting.
Symptoms include fever, headache, and myalgias (muscle pain). Most people
recover without sequelae and immunity after infection is life-long. A minority
of people infected with the virus will appear to recover (period of remission)
and then develop serious illness (period of intoxication). The disease is
called yellow fever because it causes liver failure and jaundice. Twenty to
fifty percent of people who develop liver failure from yellow fever will die.
 |
| CDC, 2012 |
Required vaccines
When my family and I moved to Iran in 1978 there were three
vaccines required for international travel: cholera, smallpox, and yellow
fever. By the time I traveled to Africa for the first time ten years later,
there were two vaccines required for international travel: cholera and yellow
fever. Now there is only one vaccine required for international travel: yellow
fever.
Yellow fever vaccine must be given by a provider with an official uniform stamp who will issue a International
Certificate of Vaccination or Prophylaxis (ICVP) to the recipient. This
certificate is required for entry to Angola, Benin, Burkina Faso, Burundi,
Cameroon, Central African Republic, Republic of the Congo, Côte d’Ivoire,
Democratic Republic of Congo, French Guiana, Gabon, Ghana, Guinea-Bissau,
Liberia, Mali, Niger, Rwanda, São Tomé and Príncipe, Sierra Leone, and Togo.
Other countries may require yellow fever immunization for
people arriving from yellow fever-endemic countries.
Travel resources
Centers for Disease Control and Prevention (CDC): Traveler's Health
U.S. Department of State: International Travel
- General precautions
- Mode of travel: health considerations
- Other travel health risks
- Specific infectious diseases involving potential health risks for travellers
- Vaccines
- CDC: Travel Clinics
Alexander, J. P., Ehresmann, K., Seward, J., Wax, G.,
Harriman, K., Fuller, S. et al. (2009). Transmission of imported
vaccine-derived poliovirus in an undervaccinated community in Minnesota.
Journal of Infectious Diseases, 199(3), 391-397. http://jid.oxfordjournals.org/content/199/3/391.full.
Apicella, M. A. (2010). Neisseria meningitidis. In G. L. Mandell,
J. E. Bennett, & R. Dolin (Eds.) Mandell, Douglas, and Bennett's principles
and practice of infectious diseases, (7th Ed.). Elsevier [Electronic version].
Centers for Disease Control and Prevention. (1994). Typhoid
immunization recommendations of the Advisory Committee on Immunization
Practices (ACIP). Morbidity and Mortality
Weekly Report, 43(14), 1-7. http://www.cdc.gov/mmwr/preview/mmwrhtml/00035643.htm.
Centers for Disease Control and Prevention. (1998). Measles,
mumps, and rubella – vaccine use and strategies for elimination of measles,
rubella, and congenital rubella syndrome and control of mumps: recommendations
of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report,
47(8), 1-57. http://www.cdc.gov/mmwr/preview/mmwrhtml/00053391.htm
Centers for Disease Control and Prevention. (2006). Imported
vaccine-associated paralytic poliomyelitis – United States, 2005. Morbidity and Mortality Weekly Report, 55(4),
97-99. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5504a2.htm.
Centers for Disease Control and Prevention. (2010). Japanese
encephalitis vaccines. Recommendations of the Advisory Committee on
Immunization Practices (ACIP). Morbidity
and Mortality Weekly Report, 59(1), 1-27. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5901a1.htm.
Centers for Disease Control and Prevention. (2012). CDC health information for international
travel 2012. New York: Oxford University Press. [Electronic version].
http://wwwnc.cdc.gov/travel/page/yellowbook-2012-home.htm.
Centers for Disease Control and Prevention. (2012). Measles
– United States, 2011. Morbidity and
Mortality Weekly Report, 61(15), 253-257. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6115a1.htm.
Jensenius, M., Han. P. V., Schlagenhauf, P., Schwartz, E.,
Parola, P., Castelli, F. et al. (2013). Acute and potentially life-threatening
tropical diseases in western travelers – a GeoSentinal multicenter study,
1996-2011. American Journal of Tropical
Medicine and Hygiene, 88(2), 397-404. http://www.ajtmh.org/content/88/2/397.full.
Jong, E. C. (2008). Immunizations for travelers. In E. C.
Jong & C. Sanford (Eds.) Travel and
tropical medicine manual. (4th Ed.). Elsevier. [Electronic version].
Steffen, R. & Grieve, S. (2013). Epidemiology: morbidity
and mortality in travelers. In J. S. Keystone, D. O. Freedman, P. E. Kozarsky,
B. A. Connor, & H. D. Nothdurft (Eds.) Travel
medicine (3rd Ed.). Elsevier. [Electronic version].
Thielman, N. M., Crump, J. A., & Guerrant, R. L. (2010).
Enteric fever and other causes of abdominal symptoms with fever. In G. L.
Mandell, J. E. Bennett, & R. Dolin (Eds.) Mandell, Douglas, and Bennett's principles and practice of infectious
diseases, (7th Ed.). Elsevier [Electronic version].
Vaughn, D. W., Barrett, A., Solomon, T. (2010). Flaviviruses
(yellow fever, dengue, dengue hemorrhagic fever, Japanese encephalitis, West
Nile encephalitis, St. Louis encephalitis, tick-borne encephalitis). In G. L.
Mandell, J. E. Bennett, & R. Dolin (Eds.) Mandell, Douglas, and Bennett's principles and practice of infectious
diseases, (7th Ed.). Elsevier [Electronic version].
Saturday, February 23, 2013
Influenza vaccine effectiveness
Holly, Andrew, and I have been settling in to our new home.
Holly has done an amazing job of setting up her new piano studio. I've been
brushing up on my (non-existent) handyman skills around our 120 year old house.
 |
| Andrew's room in our old house was too small to play on the floor |
 |
| It's time for a trim when Andrew can grab a handful of beard |
Flu vaccine recommendation for children 6 months to 8
years of age
I took Andrew in for his second flu shot this week. He
received his first dose of flu vaccine at his 6 month well-child visit last
month. The current Advisory Committee on Immunization Practices (ACIP) flu vaccine recommendation is two doses of 2012-2013 flu vaccine at least one month apart for children 6
months to 8 years of age who have not received at least two doses of flu
vaccine since July 1, 2010 and one dose of 2012-2013 flu vaccine for children
who have received at least two doses of flu vaccine since July 1, 2010.
Influenza vaccine effectiveness
The words efficacy and effectiveness have different but
related meanings in medical literature. Basically, efficacy is how well an
intervention works in clinical trials and effectiveness is how well the
intervention works in the real world. The efficacy and effectiveness of
vaccines are both calculated by comparing the number vaccinated and
unvaccinated people who develop the disease after being vaccinated.
In January the Centers for Disease Control and Prevention
(CDC) published early estimates of seasonal influenza vaccine effectiveness. In that report, the
overall vaccine effectiveness (VE) was:
Influenza A and B: 62%
Influenza A: 55%Influenza B: 70%
This week the CDC published interim adjusted estimates of seasonal influenza vaccine effectiveness. They found similar
levels of protection against influenza in people ages 6 months to 64 years, but
the effectiveness for people 65 years of age and older was not statistically significant; that is, the data they collected did not demonstrate that flu
vaccine protects older adults from influenza.
|
Influenza A & B
|
|
H3N2 (Influenza A)
|
|
Influenza B
|
|||
|
Age
|
VE
|
|
Age
|
VE
|
|
Age
|
VE
|
|
6 mos–17 years
|
64%
|
|
6 mos–17 years
|
58%
|
|
6 mos–17 years
|
64%
|
|
18–49
|
52%
|
|
18–49
|
46%
|
|
18–49
|
68%
|
|
50–64
|
63%
|
|
50–64
|
50%
|
|
50–64
|
75%
|
|
≥65
|
27% (n.s.)
|
|
≥65
|
9% (n.s.)
|
|
≥65
|
67% (n.s.)
|
|
Overall
|
56%
|
|
Overall
|
47%
|
|
Overall
|
67%
|
Last year the Center for Infectious Disease Research & Policy (CIDRAP) published a report titled The Compelling Need for Game-Changing Influenza Vaccines. The report includes an analysis of flu vaccine efficacy and effectiveness that was published in Lancet Infectious Diseases in 2011. The CIDRAP report is an excellent review of influenza and flu vaccines. The authors discuss how current influenza vaccines target the hemagglutinin head of flu viruses, which is coded by genes that mutate almost continuously. These mutations change the shape of the hemagglutinin and reduce the ability of antibodies from immunization or previous infections to bind to the virus. This is one of the reasons flu vaccines change from year to year.
The authors of the CIDRAP report call for research and
development of "universal" flu vaccines that target conserved viral
antigens; that is, vaccines that target parts of the virus that are common across
influenza virus types and strains. Research for universal flu vaccines is
ongoing, but development is likely to take another 5 to 10 years.
In the mean time, the authors of these reports remind us
that flu vaccines remain the best way to prevent influenza and its
complications.
Immunizing children to protect older adults
Because they interact with children from other households as
well as adults in their own homes, schoolchildren can facilitate disease
transmission within a community. Previous studies have
suggested that vaccinating children can protect unvaccinated people by
preventing the spread of the disease from child-to-child and then to other
households.
During the 2008-2009 flu season, Mark Loeb and colleagues gave flu vaccine to children in randomly selected Hutterite communities in Canada and, as a control, hepatitis A
vaccine to children in other Hutterite communities. The researchers found that in
the communities in which children had been given flu vaccine, the vaccine was
61% effective in preventing flu in adults who had not received the
vaccine.
Because there were few adults ages 65 years and older in the
communities Loeb et al. could not conclude that flu vaccine given to children
can protect people in that age group. Nevertheless, the Hutterite study
provided strong evidence that flu vaccine administered to children can prevent
flu in unimmunized adults.
For most people, flu vaccines are moderately effective at
preventing influenza. It's important to remember that, for most people, flu
vaccines reduce the risk of getting the flu. That means that people who receive
a seasonal flu vaccine are less likely to miss work and less likely to give the
flu to someone else. Parents of vaccinated children are less likely to miss
work staying home to take care of a sick child.
References:
Centers for Disease Control and Prevention. (2012). Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. Morbidity and Mortality Weekly Report, 61(32), 613-618. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6132a3.htm.
Centers for Disease Control and Prevention. (2013). Early
Estimates of Seasonal Influenza Vaccine Effectiveness — United States, January
2013. Morbidity and Mortality Weekly
Report, 62(2), 32-35. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6202a4.htm.
Centers for Disease Control and Prevention. (2013). Interim
Adjusted Estimates of Seasonal Influenza Vaccine Effectiveness — United States,
February 2013. Morbidity and Mortality
Weekly Report, 62(7), 119-123. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6207a2.htm.
Centers for Disease Control and Prevention. (2013). Updated
Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and
Acellular Pertussis Vaccine (Tdap) in Pregnant Women — Advisory Committee on
Immunization Practices (ACIP), 2012. Morbidity
and Mortality Weekly Report, 62(7), 131-135. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6207a4.htm.
Dushoff, J., Plotkin, J. B., Viboud, C., Simonsen, L.,
Miller, M., Loeb, M. et al. (2007). Vaccinating to protect a vulnerable
subpopulation. PLoS Medicine, 4(5),
e174. http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.0040174.
Jordan, R., Connock, M., Albon, E., Fry-Smith, A.,
Olowokure, B., Hawker, J. et al. (2006). Universal vaccination of children
against influenza: Are there indirect benefits to the community? A systematic
review of the literature. Vaccine, 24(8),
1047-1062. http://www.ncbi.nlm.nih.gov/pubmed/16298026.
Loeb, M., Russell, M. L., Moss, L., Fonseca, K., Fox, J.,
Earn, D. J. D. et al. (2010). Effect of influenza vaccination of children on
infectious rates in Hutterite communities. Journal
of the American Medical Association, 303(10), 943-950. http://jama.jamanetwork.com/article.aspx?articleid=185509.
Osterholm, M. T., Kelley, N. S., Manske, J. M., Ballering,
K. S., Leighton, T. R., & Moore, K. A. (2012). The compelling need for game-changing influenza vaccines: an analysis
of the influenza vaccine enterprise and recommendations for the future. Minneapolis:
Center for Infectious Disease Research & Policy. http://www.cidrap.umn.edu/cidrap/files/80/ccivi%20report.pdf.
Osterholm, M. T., Kelley, N. S., Sommer, A., & Belongia,
E. A. (2011). Efficacy and effectiveness of influenza vaccines: a systematic
review and meta-analysis. Lancet
Infectious Diseases, 12(1), 36-44. http://www.thelancet.com/journals/laninf/article/PIIS1473-3099(11)70295-X/abstract.
Roos, R. (2013). FDA expert: universal flu vaccine still
5-10 years off. CIDRAP News. http://www.cidrap.umn.edu/cidrap/content/influenza/general/news/feb1313hearing.html.
Weycker, D., Edelsberg, J.,
Halloran, M. E,. Longini, I. M., Nizam, A., Ciuryla, V. et al. (2005).
Population-wide benefits of routine vaccination of children against influenza. Vaccine, 23(10), 1284-1293. http://www.ncbi.nlm.nih.gov/pubmed/15652671.
Subscribe to:
Posts (Atom)












